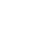The Apple Watch’s atrial fibrillation (AFib) history feature has been qualified by the FDA under its Medical Device Development Tools (MDDT) program, the first digital health technology feature of its kind to do so.
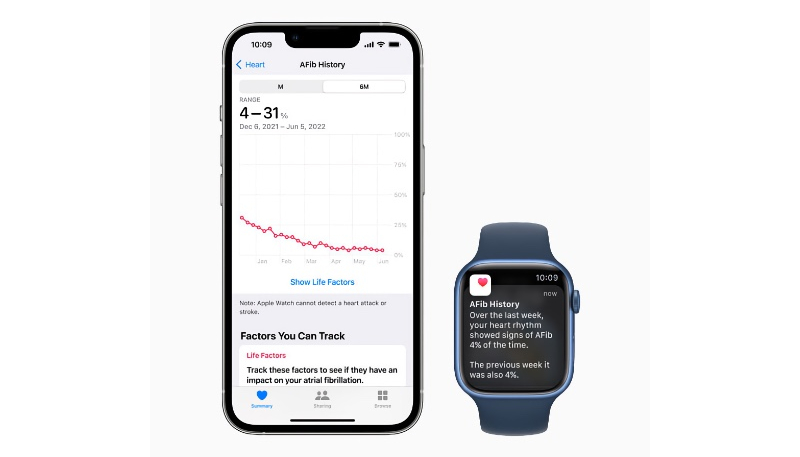
The Apple Watch has supported AFib History since 2022 and allows users with atrial fibrillation to view an estimate of how often their heart is beating in this type of irregular rhythm.
From Apple’s website:
About AFib and AFib History
Atrial fibrillation (AFib) is a type of irregular heart rhythm where the upper chambers of the heart beat out of sync with the lower chambers.
According to the CDC, approximately 2% of people younger than 65 years old and 9% of people 65 and older have AFib. Irregularities in heart rhythm become more common as people get older. Some individuals with AFib don’t experience any symptoms. Others experience symptoms that could include rapid heartbeat, palpitations, fatigue, or shortness of breath.
AFib is a chronic condition, but the amount of time people spend in AFib can change. People with AFib often live healthy, active lives. The amount of time your heart is in AFib can potentially be reduced with regular exercise, a heart-healthy diet, a healthy weight, and treatment of other medical conditions that could worsen AFib. If left untreated, AFib can lead to heart failure or blood clots that may lead to stroke.
AFib History gives long-term visibility into the amount of time your heart shows signs of AFib, also known as AFib burden, so you can share this information with your physician for richer conversations.
Apple says the feature is intended for use by individuals aged 22 years or older who have been diagnosed with atrial fibrillation.
The MDDT program under which the AFib History feature was approved is the FDA’s method of qualifying tools that medical device sponsors can choose to use in the development and evaluation of medical devices.
The FDA says the Apple Watch is the first digital health technology qualified under the MDDT program and provides a non-invasive way to check estimates of atrial fibrillation (AFib) burden within clinical studies.